The Science Behind Drug Action
Medical professionals, researchers, and especially pharmaceutical sales representatives can benefit from understanding how drugs work in the body. Their role involves presenting complex drug information to healthcare professionals who rely on this knowledge to make informed prescribing decisions. They could also better list a drug’s benefits over its competitors. Learning pharmacokinetics (PK) and pharmacodynamics (PD) concepts can give sales reps a better understanding of how drugs reach therapeutic levels while minimizing risks.
Imagine a patient who takes ibuprofen to treat a headache. After swallowing the pill, it dissolves in the stomach and passes through the intestines where it gets absorbed. Once in the bloodstream, it is transported to its target tissues, inhibiting enzymes (like COX-2) that cause pain and inflammation.2 Eventually, the liver metabolizes the drug, and the kidneys excrete it via urine. This entire process illustrates how PK and PD work together.
A drug’s solubility, how the liver metabolizes it, and patient-specific factors like genetics can influence the journey of a drug. For example, a patient with CKD (chronic kidney disease) will have diminished clearance of medications that require the kidney to be excreted; if dose adjustments based on this altered clearance are not made, they would be at high risk for drug accumulation and toxicity. These details are the reasons why knowledge of PK and PD is fundamental to making every drug both effective and safe for every patient.3
Pharmacokinetics
PK focuses on what the body does to a drug. It has four main steps:
- Absorption: The drug enters the bloodstream from the site of administration. Factors like the formulation (liquid vs. tablet), route (oral vs. IV), and the patient’s gastrointestinal health (gastric pH) can influence how quickly this happens.2
- Distribution: Following absorption, the drug is delivered to other parts of the body (cells/tissues). For instance, highly lipophilic drugs have an easier time crossing the blood-brain barrier, eliciting effects on the central nervous system, while others may remain mostly in the bloodstream.3
- Metabolism: A process by which the body can detoxify or transform drugs or chemicals. Metabolism can activate or inactivate drugs by modifying its chemical structure. A common example is how codeine must be metabolized into morphine for pain relief.2
- Excretion: The drug and its metabolites are removed from the body, primarily via urine or bile but in smaller quantities through saliva, breast milk, and sweat. Impaired kidney function can slow this process, requiring dose adjustments to prevent toxicity.3
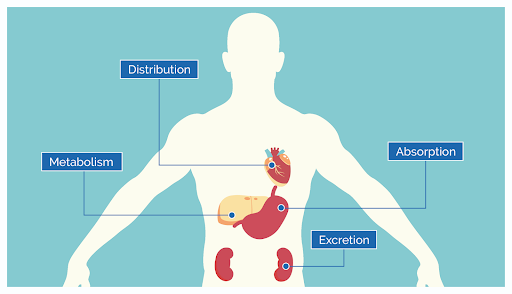
Together, these processes determine the drug’s concentration in the bloodstream and tissues over time, directly impacting its effectiveness and safety.
Pharmacodynamics
PD examines what a drug does to the body. Key concepts include:
- Receptor Binding: Most drugs act by binding to specific receptors that cause a biological response to occur. Beta-blockers reduce heart rate and blood pressure; to “work,” these agents must bind to adrenergic receptors in the body.2
- Dose-Response: The drug’s concentration at the target location typically determines the response there. There is frequently a threshold, beyond which raising the dosage has no further advantages and only raises the possibility of adverse effects.3
- Therapeutic Index: The proportion between an effective dose and a toxic dose. Therapeutic drug monitoring is used to obtain peak and trough levels to determine drug serum concentration which tells us if a drug is efficacious or is leading to toxic levels. A wider therapeutic index, as seen with drugs like amoxicillin, offers more flexible dosing compared to drugs like warfarin, which require precise monitoring.2
Pharmacodynamics helps us understand how a drug works, how much it is needed for the desired effect, and what potential risks or adverse effects may be associated with its use.
The PK-PD Interaction: A Dynamic Duo
Pharmacokinetics determines the drug’s availability at the site of action, while pharmacodynamics determines its biological impact. For example, a drug with poor absorption (PK) may not reach therapeutic levels, regardless of its potency (PD). A highly effective drug (PD) may pose safety concerns if it accumulates due to slow metabolism or excretion (PK).
The relationship is crucial in:
- Dosing: Ensuring the right amount of drug provides benefits without causing harm, such as adjusting vancomycin doses to prevent nephrotoxicity.3
- Managing Side Effects: Balancing drug exposure and effect to achieve desired outcomes. This is especially critical in oncology, where chemotherapeutic agents require precise dosing to improve survival while minimizing side effects.2
- Combination Therapies: Understanding PK-PD relationships helps in predicting drug-drug interactions and adjusting treatment regimens accordingly.3
Emerging Trends and Applications
Technological and research developments widen the limits of PK/PD. In personalized medicine, genetic information is used to tailor treatment regimens.
- Pharmacogenomics: Treatments can be customized to meet the needs of each patient by examining how genes affect drug response. For instance, genetic testing for CYP2C19 enzyme activity determines the appropriate dosage of clopidogrel.4
- Biologics: monoclonal antibodies pose PK challenges due to their large size and complex metabolism. Equally important is understanding PD to optimize the therapeutic effects against specific molecular targets, for example, tumor necrosis factor (TNF) in the treatment of autoimmune disorders.5
Patient-Specific Factors
Individual characteristics significantly influence how drugs are processed and work:
- Age: due to reduced drug clearance, neonates and infants require lower doses of medication to avoid toxicity (e.g. aminoglycosides). Similarly, the elderly population has an increased risk of accumulation due to slowed drug metabolism and excretion (e.g. benzodiazepines).6
- Weight: underweight and obese patients may require dose adjustments due to altered volumes of distribution. Weight-based medications, like vancomycin, are usually based on adjusted body weight versus actual body weight to ensure efficacy without toxicity.6
- Kidney: kidney function is measured by creatinine clearance (CrCl) or estimated glomerular filtration rate (eGFR). Reduced kidney function affects the elimination of really excreted drugs, like aminoglycosides. Drug doses and/or intervals need to be adjusted to prevent accumulation and toxicity.6
- Liver: liver function is determined by liver function tests (LFTs), which measure the levels of enzymes and proteins made by the liver. The impaired liver function reduces the metabolism of drugs processed by hepatic enzymes (e.g. warfarin) which leads to higher plasma concentrations.6
- Comorbidities: patients diagnosed with diabetes have the possibility of developing gastroparesis also known as delayed gastric emptying which can slow the absorption of drugs taken orally (e.g. metformin).6
Relevance in Pharmaceutical Roles
A strong understanding of PK and PD is extremely useful for pharma sales reps because this knowledge fosters more informed conversations with HCPs, increasing the likelihood of successful communication about their products. Understanding a drug’s onset of action, duration, and therapeutic effects helps reps to align product benefits with patient needs effectively. For sales representatives, the PRC and CSPP certifications can enhance this understanding and communication with HCPs:
- Pharmaceutical Representative Certification (PRC): This certification provides professionals with a foundational knowledge of pharmacology, regulatory standards, and effective communication strategies. A solid grasp of PK and PD is essential for explaining drug benefits and safety to healthcare professionals.1
- Certified Specialty Pharma Professional (CSPP): This certification dives deeper into the clinical and scientific aspects of pharmaceuticals, including more complex products such as biologics and biosimilars.
Ensuring Therapeutic Success
An understanding of PK and PD is essential to ensuring therapeutic success. By learning these concepts, healthcare professionals can:
- Develop safer and more effective drugs.
- Tailor treatments to individual patient needs.
- Reduce the risks of adverse effects and drug interactions.
- Improve communication between healthcare providers, patients, and pharmaceutical stakeholders.
PK and PD continue to be essential for drug development and patient care in the era of personalized medicine and advanced therapeutics. These principles guide strategic decisions for healthcare policies and resource distribution, ensuring that treatments are both clinically effective and cost-effective. 7
Conclusion
The journey of a drug demonstrates the balance of PK and PD. Expanding our knowledge of how drugs work allows us to promote advancements in therapy, research, and patient care. For sales professionals in the pharmaceutical industry, certifications like PRC and CSPP provide opportunities to improve their knowledge and excel in their careers. By applying the principles of PK and PD, we can continue to make advancements in healthcare and improve patient outcomes.
References
- ACMALifeSciences. “Training and Certification Programs.” Accessed January 3, 2025. https://acmalifesciences.org/training
- Rang, H. P., Dale, M. M., Ritter, J. M., Flower, R. J., & Henderson, G. (2019). Rang & Dale’s Pharmacology. 9th Edition. Elsevier. pp. 4-32, 117-162.
- Goodman, L. S., & Gilman, A. G. (2021). The Pharmacological Basis of Therapeutics. 13th Edition. McGraw-Hill Education. pp. 14-51.
- T P A, Sekhar S, Jose A, et al. Pharmacogenomics: The Right Drug to the Right Person. Journal of Clinical Medicine Research. 2009;1(4). doi:https://doi.org/10.4021/jocmr2009.08.1255
- Kothari M, Wanjari A, Acharya S, et al. A Comprehensive Review of Monoclonal Antibodies in Modern Medicine: Tracing the Evolution of a Revolutionary Therapeutic Approach. Cureus. 2024;16(6). doi:https://doi.org/10.7759/cureus.61983
- Mangoni AA, Jackson SHD. Age-related Changes in Pharmacokinetics and Pharmacodynamics: Basic Principles and Practical Applications. British Journal of Clinical Pharmacology. 2004;57(1):6-14. doi:https://doi.org/10.1046/j.1365-2125.2003.02007.x
- Srinivasan M, White A, Chaturvedula A, et al. Incorporating Pharmacometrics into Pharmacoeconomic Models: Applications from Drug Development. Pharmacoeconomics. 2020;38(10):1031-1042. doi:https://doi.org/10.1007/s40273-020-00944-0
- Gleichmann N. What Is ADME? Drug Discovery from Technology Networks. Published June 26, 2020. https://www.technologynetworks.com/drug-discovery/articles/what-is-adme-336683 (image)


